Main navigation
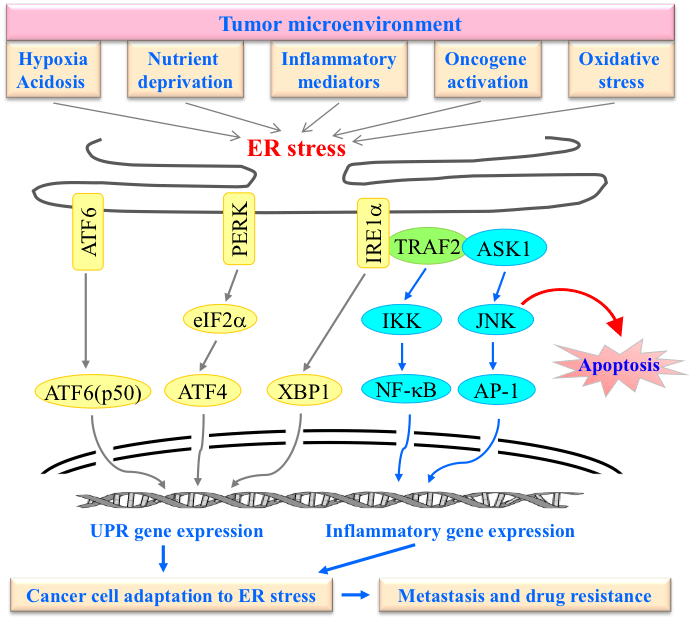
Tumor microenvironment is characterized by hypoxia, low glucose and free radicals, and known to trigger chronic inflammation and endoplasmic reticulum (ER) stress (Fig. 3). Adaptation to this milieu has profound consequence for cancer cell survival, metastasis and response to therapy. We recently discovered that TRAF2 protects mammary epithelial cells (MECs) from apoptosis during lactation, and that TRAF2 expression is essential for the development and growth of MMTV-Neu-induced mammary tumor in mice. Exposure of MECs and breast cancer (BC) cells to various stress-inducing agents in vitro revealed that TRAF2 expression and phosphorylation are essential for the survival of BCs under ER stress conditions. Bioinformatics analysis reveals that TRAF2 is overexpressed and constitutively phosphorylated in primary BCs, and this overexpression is associated with poor prognosis in patients with invasive breast carcinoma. Currently, we are investigating the molecular mechanisms by which TRAF2 expression and phosphorylation promotes cancer cell survival under ER stress conditions.