Main navigation
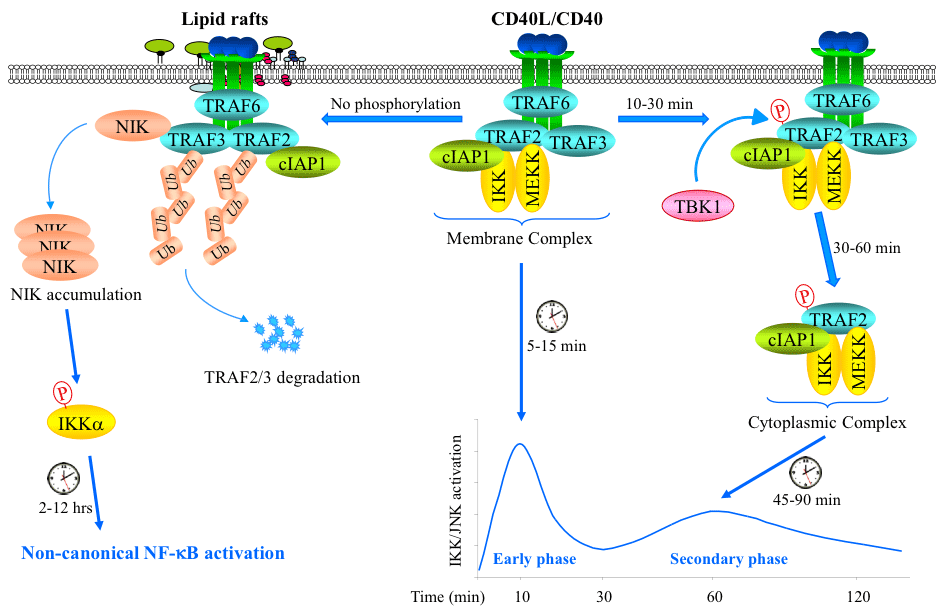
The CD40L/CD40 dyad regulates B cell proliferation, differentiation, and survival to promote humoral immunity. Ligation of CD40 activates the MEKK-JNK and canonical IKK-IkBα-NF-κB (mainly the p50/RelA dimer) pathways within 10-60 min, and the noncanonical NIK-IKKα-NF-κB (mainly the p52/RelB dimer) pathway 2-12 hrs after stimulation (Fig. 2); however, the underlying mechanisms have been elusive. We discovered that CD40L stimulation elicits TRAF2 phosphorylation in B cells, and that this phosphorylation is required for the translocation of the signaling complex from CD40 to the cytoplasm to activate the prolonged phase of JNK and IKK. In the absence of TRAF2 phosphorylation, the signaling complex translocates to the lipid rafts where TRAF2 and TRAF3 are degraded, which allows NIK protein to accumulate and activate the noncanonical NF-κB pathway. In addition, we found that TRAF2 is constitutively phosphorylated in a subset of B cell-derived cancer cell lines and primary tumor samples, and that this phosphorylation promotes lymphoma cell survival under conditions of cellular stresses. These data suggest that TRAF2 phosphorylation functions as a molecular switch to control the spatiotemporal activation of the canonical and noncanonical NF-κB pathways, and that TRAF2 phosphorylation is one of the mechanisms responsible the constitutive activation of NF-κB in B-cell lymphomas. Currently, we are investigating how TRAF2 phosphorylation regulates the cytoplasmic translocation of the CD40 signaling complex and lymphoma survival.