Main navigation
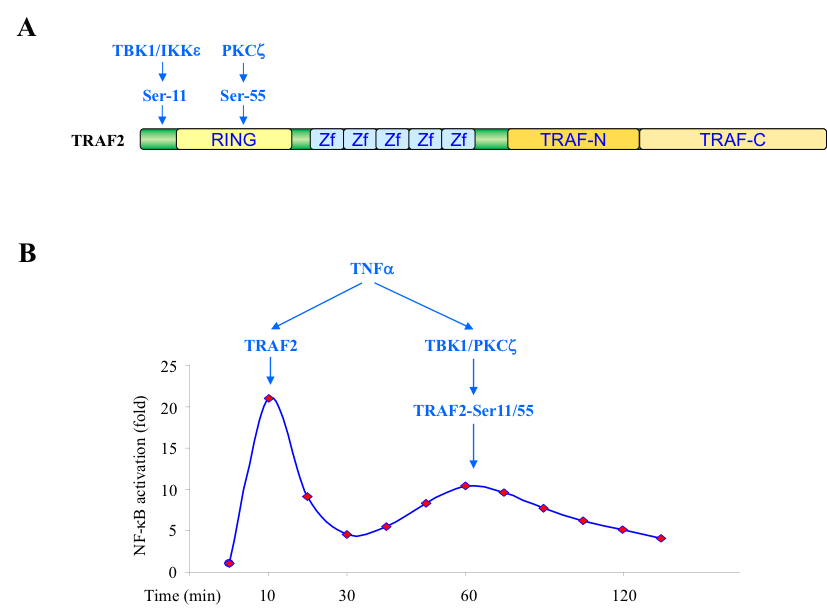
TNFα is a key modulator of inflammatory responses and necroptosis (a programmed form of necrosis), and exerts its biological effects by activating the pro-survival (TRAF2/RIP1/IKK/NF-κB), pro-apoptotic (RIP1/FADD/Caspase-8) and pro-necroptotic (RIP1/RIP3/MLKL) pathways. TNFα production is elevated in immune-mediated inflammatory diseases (IMIDs), and thereby targeted in therapeutic strategies for a range of IMIDs. Despite the therapeutic efficacy of TNFα antagonists in treating some IMIDs (e.g. rheumatoid arthritis), TNFα blockade has an unpredictable outcome in others, leading to inappropriate activation of immune response in many cases, including manifestations of lupus and increased production of autoantibodies. Thus, identification of additional molecular events that modulate TNFα signaling is an important task for a better understanding of the pathogenesis of IMIDs and the development of novel therapeutic interventions. We mapped two phosphorylation sites, Ser-11 and Ser-55, in the N-terminal region of TRAF2, and identified TBK1 and PKCζ as the kinases that directly phosphorylate TRAF2 at these sites (Fig. 1A). Functional studies revealed that TRAF2 phosphorylation promotes the secondary/prolonged phase of IKK activation that occurs between 45 and 75 minutes after TNFα stimulation, but has no effect on the immediate phase of IKK activation that take place within 3-10 min (Fig. 1B). Currently, we are investigating how TRAF2 phosphorylation regulates the pronged phase of IKK activation and inflammatory responses.